Navigating the Complexities of Medical Device Software: An Exploration of MAP-R Testing for MCPS
Related Articles: Navigating the Complexities of Medical Device Software: An Exploration of MAP-R Testing for MCPS
Introduction
With great pleasure, we will explore the intriguing topic related to Navigating the Complexities of Medical Device Software: An Exploration of MAP-R Testing for MCPS. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Navigating the Complexities of Medical Device Software: An Exploration of MAP-R Testing for MCPS
The realm of medical devices is inherently complex, demanding rigorous testing to ensure the safety and efficacy of these life-saving tools. Within this critical domain, medical device software (MDS) plays an increasingly pivotal role. As the functionality of medical devices becomes more sophisticated, so too does the need for comprehensive and robust testing methodologies. One such methodology, gaining significant traction in the industry, is MAP-R testing, a structured approach specifically designed to evaluate the performance and reliability of MCPS (Medical Computer Systems).
Delving into the Nuances of MAP-R Testing
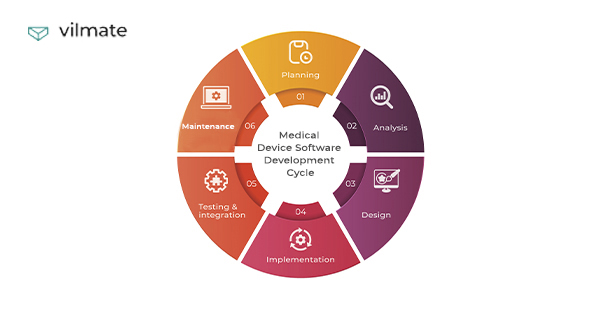
MAP-R testing, an acronym for Manufacturer Acceptance Process for Release, signifies a comprehensive set of procedures meticulously designed to validate the readiness of MCPS for market release. This methodology encompasses a multi-faceted approach, encompassing various stages of testing, from the initial development phase to the final validation before deployment.
The Core Pillars of MAP-R Testing
MAP-R testing is characterized by its structured and systematic nature, adhering to a defined set of principles:
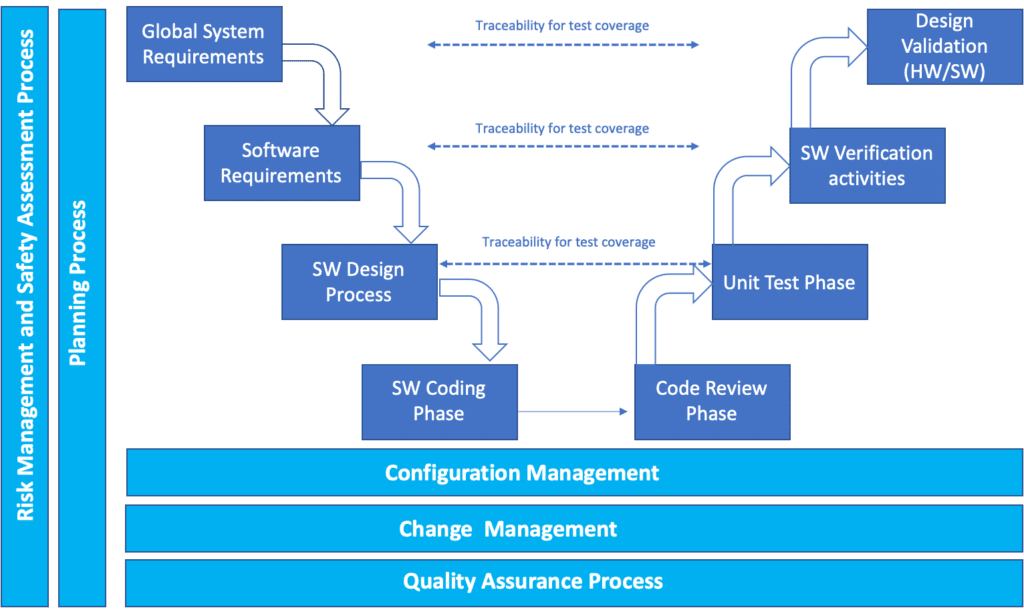
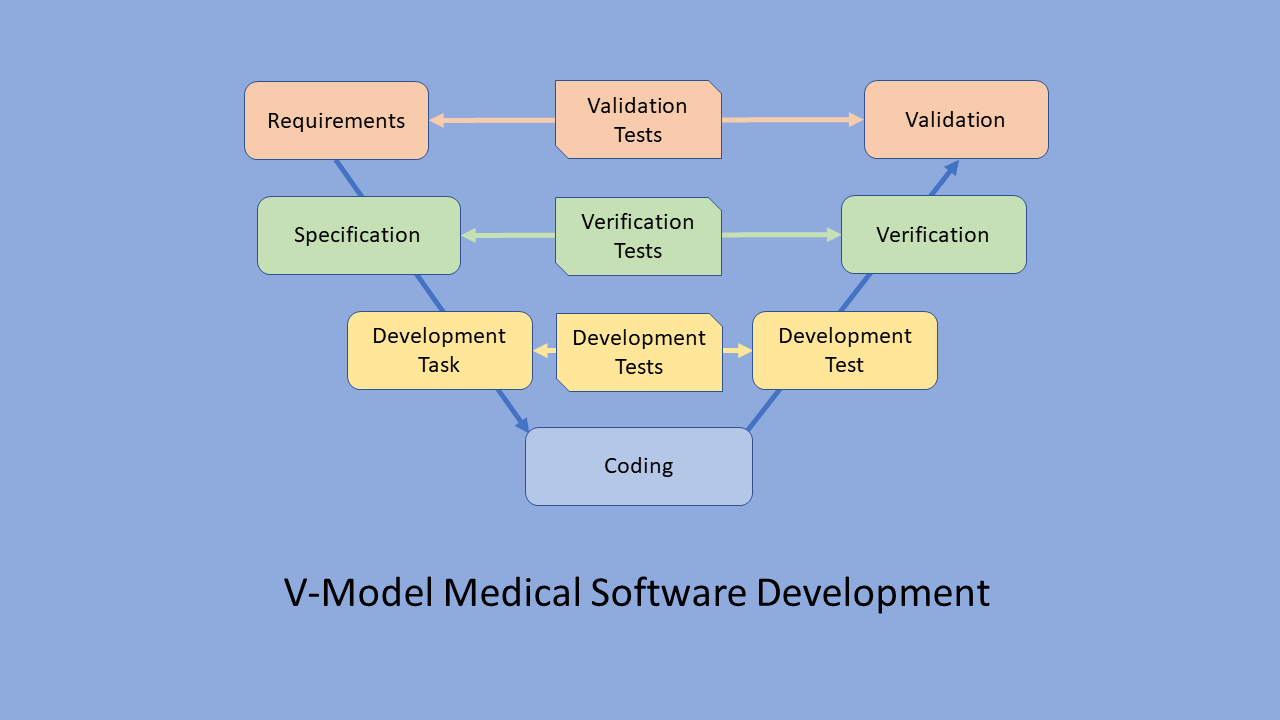
- Requirements Verification: The process begins with a thorough examination of the MCPS’s requirements, ensuring that the software aligns with the intended purpose and adheres to regulatory guidelines. This involves meticulously reviewing specifications, user stories, and functional requirements.
- Design Validation: Once the requirements are established, the design of the MCPS undergoes rigorous scrutiny. This phase involves validating the architecture, algorithms, and user interfaces to ensure they meet the specified requirements and function as intended.
- Code Review and Unit Testing: MAP-R testing emphasizes the importance of code quality. Thorough code reviews and unit testing are conducted to identify potential defects, improve code readability, and ensure that individual components of the software function as expected.
- Integration Testing: As individual software components are developed, integration testing ensures that these components seamlessly interact with each other and function correctly as a cohesive system. This phase helps identify compatibility issues and ensures smooth data flow between different parts of the software.
- System Testing: Once the MCPS is fully integrated, system testing simulates real-world scenarios, evaluating the software’s performance, functionality, and usability under various conditions. This comprehensive testing phase assesses the software’s ability to handle expected and unexpected inputs, ensuring its reliability and stability.
- Performance Testing: Performance testing focuses on the software’s ability to handle high volumes of data, process requests efficiently, and maintain responsiveness under stress. This phase evaluates the software’s scalability and its ability to deliver consistent performance even during peak usage.
- Usability Testing: MAP-R testing prioritizes the user experience. Usability testing involves evaluating the software’s intuitiveness, ease of use, and overall user satisfaction. This phase ensures that the software is user-friendly and meets the needs of its intended audience.
- Security Testing: In the context of medical devices, security is paramount. Security testing assesses the MCPS’s vulnerability to cyberattacks, data breaches, and unauthorized access. This phase ensures the software’s ability to protect sensitive patient information and maintain data integrity.
- Regulatory Compliance Testing: MAP-R testing incorporates compliance testing to ensure that the MCPS adheres to all relevant regulatory standards and guidelines. This may involve testing for compliance with ISO 13485, IEC 62304, and other applicable regulations.
The Significance of MAP-R Testing
MAP-R testing plays a pivotal role in the development and deployment of MCPS, offering numerous benefits:
- Enhanced Safety and Efficacy: By thoroughly testing the software, MAP-R testing helps ensure the safety and efficacy of medical devices. This reduces the risk of software errors that could lead to patient harm or compromised treatment outcomes.
- Improved Software Quality: The rigorous testing processes inherent in MAP-R testing contribute to the development of high-quality software, minimizing defects and enhancing the overall reliability of the MCPS.
- Reduced Development Costs: While initial testing may seem costly, MAP-R testing helps identify and resolve defects early in the development cycle, reducing the risk of costly rework and delays later in the process.
- Increased Patient Confidence: By demonstrating the software’s reliability and safety, MAP-R testing fosters trust and confidence in the use of medical devices among patients and healthcare professionals.
- Compliance with Regulations: MAP-R testing ensures compliance with relevant regulatory standards, facilitating the successful market release of the MCPS and avoiding potential legal issues.
FAQs about MAP-R Testing for MCPS
Q: What are the key differences between MAP-R testing and traditional software testing methodologies?
A: MAP-R testing is specifically tailored for medical device software, incorporating a focus on safety, efficacy, and regulatory compliance that may not be as prominent in traditional software testing methodologies. Additionally, MAP-R testing often involves more extensive documentation and traceability requirements.
Q: How can organizations effectively implement MAP-R testing into their development processes?
A: Successful implementation of MAP-R testing requires a dedicated team of skilled testers, a well-defined testing strategy, and appropriate tools and infrastructure. It is also crucial to integrate MAP-R testing into the software development lifecycle (SDLC) from the very beginning.
Q: What are the potential challenges associated with MAP-R testing?
A: Challenges may arise from the complexity of medical device software, the need for specialized expertise, and the strict regulatory requirements that must be met. Organizations should proactively address these challenges through careful planning, resource allocation, and ongoing training.
Tips for Effective MAP-R Testing
- Establish a Clear Testing Strategy: Define the scope, objectives, and methodology of the MAP-R testing process to ensure a structured and efficient approach.
- Utilize Appropriate Testing Tools: Leverage specialized testing tools designed for medical device software to automate repetitive tasks, enhance test coverage, and improve efficiency.
- Foster Collaboration: Encourage collaboration between developers, testers, and regulatory experts to ensure a comprehensive and effective testing process.
- Document Thoroughly: Maintain detailed documentation of all testing activities, including test cases, results, and any identified defects.
- Continuously Improve: Regularly review and refine the MAP-R testing process to identify areas for improvement and ensure its ongoing effectiveness.
Conclusion
MAP-R testing is an indispensable methodology for ensuring the safety, efficacy, and reliability of MCPS. By meticulously testing the software throughout the development lifecycle, this approach helps mitigate risks, enhance software quality, and foster confidence in the use of medical devices. As the role of software in healthcare continues to expand, the importance of MAP-R testing will only grow, ensuring that medical devices remain safe and effective tools for improving patient care.




Closure
Thus, we hope this article has provided valuable insights into Navigating the Complexities of Medical Device Software: An Exploration of MAP-R Testing for MCPS. We thank you for taking the time to read this article. See you in our next article!

